German researchers have developed a novel electro-organic synthesis that enables chemists to work with the available electricity supply and use a wide variety of non-specialized equipment.
Electrochemistry is an old discipline. Hermann Kolbe (1818-1884), a German organic chemistry pioneer, developed the “Kolbe process”, aka the Kolbe–Schmitt reaction, a chemical reaction carried out by electricity, more than 160 years ago.
However, the long history of electrochemical syntheses has not been translated into tangible commercial realities within the chemical production industry.
Several research projects are aimed at advancing the techniques and equipment for electrochemists. And, in recent years, commercial applications of electrolysis have begun to develop, for the manufacture of fine organic products for perfumery, pharmacy, and cosmetology, among other industries.
Novel Electro-Organic Synthesis for Green and Sustainable Chemical Industry
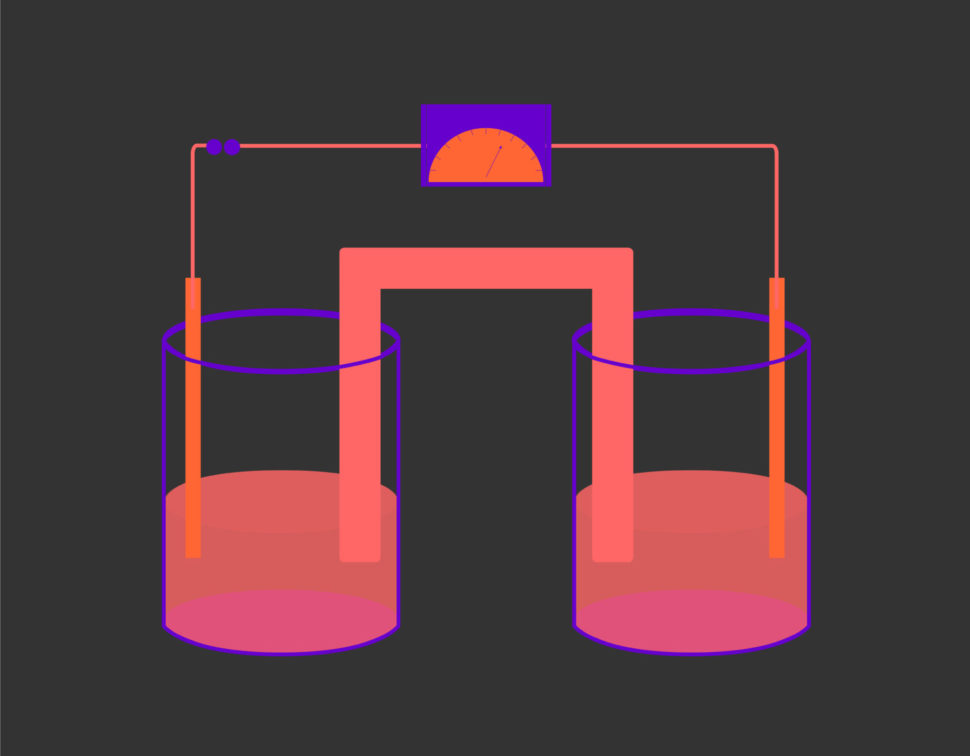
Electrosynthesis processes usually take a long time during which they have to be highly controlled using custom-made equipment that requires uniform electricity input.
In addition to reducing costs, electrochemistry also reduces environmental pressures on the chemical industry, by using surplus energies from wind, solar, and other renewable sources.
As part of EPSYLON (a cooperative research project funded by the German Federal Ministry of Education and Research), a joint team of researchers from Johannes Gutenberg University (Mainz) and Evonik Performance Materials reported a breakthrough in electro-organic synthesis.
EPSYLON coop research project improves electro-organic synthesis.Click To TweetThe novel electrolyte system allows green production of fine chemicals and will help make the chemical industry more eco-friendly and sustainable. Compared to other reagents, reducing metals or strong oxidants, the electron as a reagent is cheaper and less toxic.
What’s more, with this method, operators won’t have to use complex customized electrolysis equipment that requires constant current-density. To carry out synthesis reactions, they will have at their disposal a wide range of devices and can rely on the surplus power from renewables.
The Evonik/JGU team used electrolyzes that can work within a tighter current-density window while retaining high selectivity and productivity.
“This allows variations of the current density of more than two orders of magnitude without decreasing selectivity or product yield,” said researchers in the abstract of the study published in the journal Science Advances. “It potentially paves the way for industrial electrolyzers with variable current consumption, which could enable the flexible use of energy surplus in the electricity supply.”


















Comments (0)
Most Recent