Researchers just created a new chemical composite that can allegedly capture, store, and use heat from the Sun.
A new chemical composite developed by researchers from the Massachusetts Institute of Technology could provide an alternative solution to capturing and storing thermal energy from the Sun.
In far-flung places outside the power grid, especially remote areas in developing countries, communities primarily depend on the abundant supply of heat and light from the Sun to do their day to day activities. However, nighttime brings in a different story.
Without the Sun, people in developing areas scour for wood, brush, or dung that they can use as fuel to light their homes and keep them warm. Such activity requires a tremendous amount of time and effort, particularly during the cold and wet season.
But, thanks to this latest innovation from MIT, things might be about to change.
#MIT just created a new chemical composite for thermal energy storage.Click To TweetThe new Chemical Composite: How it Works
Typically, storing thermal energy requires the usage of an approach known as phase change material. With the PCM approach, input heat melts the material and changes phase from solid to liquid. When PCM cools down below its melting point, it will turn back to solid, at which point the stored energy will be released as heat.
Similar phase change materials make use of fatty acids or waxes in low-temperature applications and molten salts in high-temperature applications. Sometimes, molten salts are used in combination with concentrated solar plants that work by using reflected sunlight. The process melts the salts which then power conventional steam turbines.
However, current PCMs require a great deal of insulation. Aside from that, they pass through the phase change temperature uncontrollably, resulting in a rapid loss of stored heat.
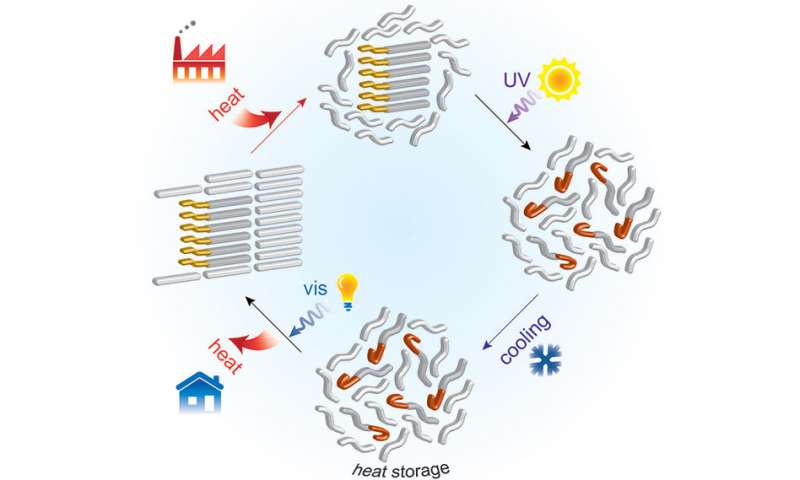
As an alternative, MIT’s new chemical composite or hybrid material uses molecular switches that could change shape in response to light. It is made possible by combining fatty acids with an organic compound that reacts to a pulse of light. The light-sensitive component then changes the thermal properties of the other component which triggers the storage and release of energy.

Apparently, the hybrid material melts when heated. However, it remains melted even when cooled down after being exposed to ultraviolet light. Then, the material returns to its solid state after being triggered by another pulse of light. Jeffrey Grossman, one of the MIT researchers, explained:
“By integrating a light-activated molecule into the traditional picture of latent heat, we add a new kind of control knob for properties such as melting, solidification, and supercooling.”
According to the researchers, they developed the new chemical composite as an add-on for traditional phase change materials. The only trick is to find a method to incorporate the molecules with current PCMs to release the stored energy as heat when needed.
“The availability of waste heat is widespread, from industrial processes, to solar heat, and even the heat coming out of vehicles, and it’s usually just wasted. What we are doing technically is installing a new energy barrier, so the stored heat cannot be released immediately,” Grace Han, another MIT researcher, said.
While the new chemical composite is still far from being commercially scalable, its potential is undeniable according to the researchers. In fact, it could be used to store heat from any source, releasing it when needed. Something that would be useful when cooking or heating after dark.
“There are so many applications where it would be useful to store thermal energy in a way lets you trigger it when needed,” Grossman went on to say.
“Our interest in this work was to show a proof of concept. But we believe there is a lot of potential for using light-activated materials to hijack the thermal storage properties of phase change materials.”












Comments (0)
Most Recent