Proton-exchange Membrane Fuel Cells (PEMFCs) are considered a valuable alternative energy technology capable of effeciently generating clean power. If these kinds of fuel cells can be made smaller and more cost-effective, they could potentially replace Li-ion batteries.
Scientists at Liverpool University have figured out a way to more efficiently transport proton charges in PEMFCs with the potential to make fuel cells a commercially viable energy option.
A fuel cell converts hydrogen and oxygen into water. Therefore, a fuel cell is an electrochemical energy conversion device that produces power, water, and heat as byproducts.
Unlike batteries, fuel cells do not require charging and unlike convention fossil fuels, fuel cells are a zero-emission source of energy.
Why Conventional Fuel Cells Fall Short
PEM applications are a class of fuel cells that use Proton-exchange membrane fuel cells, or PEMFCs.
In PEMFCs, the proton exchange membrane carries positively charged protons from the positive electrode (cathode) to the negative one (anode).
The current issue impeding the development of better PEM materials is that scientists are currently unable to study how protons move inside the PEM.
This is due to the nature of amorphous polymers, the materials currently used to make PEMs.
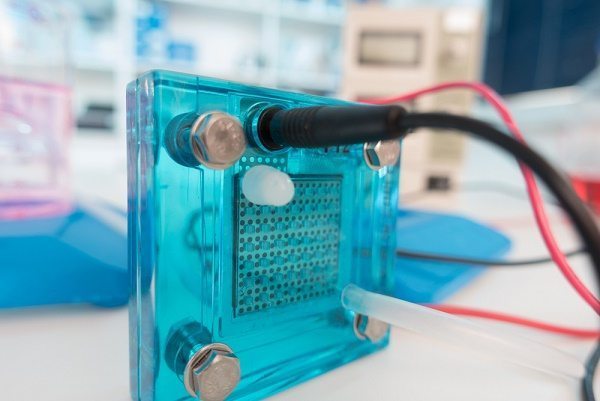
Therefore, to more efficiently transport proton charges in PEM fuel cells, scientists at Liverpool University have proposed synthesized nanometre-sized cage molecules.
Researchers synthesized molecules that enclose an internal cavity. This external layer of molecules forms a porous organic cage into which other, smaller molecules such as water or CO2 can be loaded.
When the cages form solid materials, the recepticles can arrange to form channels in which the small ‘guest’ molecules can travel from one cage to another.
“The new materials exhibited proton conductivities of up to 10-3 S cm1, which is comparable to the best porous materials.”
These synthesized molecules are a better material for use in fuel cells because they form crystals in which cages regularly arrange themselves.
This property allows researchers to build an unambiguous description of the structure using crystallography – a technique that allows scientists to pinpoint the exact location of certain atoms.
The molecules are also soluble in common solvents, which means that they could be combined with other materials to be made into membranes.
Scientist Successfully Create Better Fuel Cells
The new materials exhibited proton conductivities of up to 10-3 S cm1, which is comparable to the best porous materials available.
The cells were built so that protons can move through the cage membranes at a more rapid pace.
General Electric developed PEMFCs in the 1960s, but their use is restricted due to high costs and unnecessarily large size.
This latest development, however, means PEMFCs may not only become more cost-effective, but also small enough to replace batteries in cars and even consumer electronics such as phones.

















Comments (0)
Most Recent