A research team from Rice University in Houston may have just stumbled upon a way of improving lithium-ion battery life.
While I attended the University of Houston, I have to show love to fellow Houston university Rice — especially when one of their teams accomplishes something amazing.
Our coverage first started with the AI known as Bayou that uses deep learning to write code. Now, it moves onto batteries and…nanotubes?
What is this new breakthrough and what does it mean for battery technology?
Carbon Nanotubes Help Stop Dendrite Growth
We covered the issues facing battery technology recently with a specific focus on dendrites.
While both graphene and saltwater batteries provide alternatives, finding a solution for lithium-ion battery decay may be preferred in the long term.
In a press release published on October 25th, Rice University showcased new research into just this kind of solution. As a result of research into a cost-effective solution, the team believes they solved the dendrites issue.
The method “quenches” lithium metal dendrites for faster charging and longer charges.
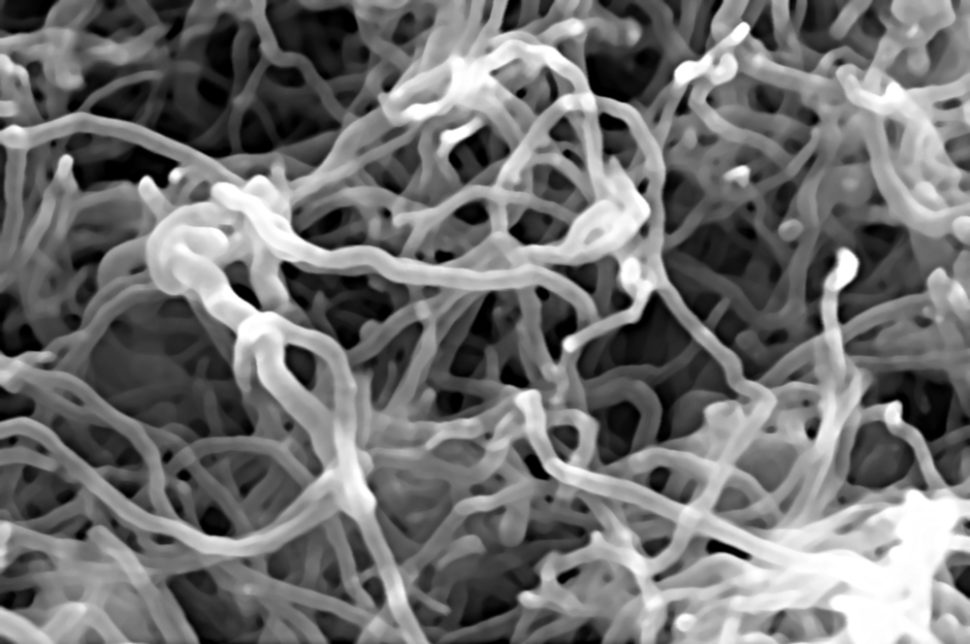
In the photo above, you can see two different types of lithium metal anodes. On the left, the anodes are protected by carbon nanotubes. On the right, it’s just the bare anodes with visible dendrite growth.
The thin nanotubes help to stop natural dendrite growth in unprotected lithium metal anodes found in batteries. It is these dendrites that spear electrolyte cores and damage the cathode. This, in turn, commonly causes batteries to fail.
But solving the dendrite issue required a balancing act when it came to charging speed.
After all, no one likes to have to wait for their phone battery to recharge. I have too much Candy Crush to play don’t you know!
But the solution, as the Rice team discovered, is inexpensive and highly effective.
New Life for Lithium Batteries From Simple Carbon
Essentially, the group of Rice researchers coated lithium metal foil with some multiwalled carbon nanotube film. James Tour, chemist and Rice affiliate, spoke about how the coating helps prevent dendrite growth.
“The lithium dopes the nanotube film, which turns from black to red, and the film, in turn, diffuses the lithium ions.” Postdoctoral researcher Rodrigo Salvatierra and graduate student Gladys López-Silva echoed the simplicity of the solution.
“The ions distribute themselves throughout the nanotube film.” As a result of this quenching process, dendrite growth does not occur as lithium anodes discharge.
The researchers showed a 99.8% retention rate of lithium metal cell coulombic efficiency. For those unfamiliar with the term (like me), this is how you measure how well the electrons move in an electrochemical system.
You can find the full abstract online right here.
With this breakthrough, we take another step in the journey to off-grid and sustainable energy storage. We also maintain faster charging and longer lasting charges (woo!).















Yes, proven by the non-toxic saltwater battery, with no hazardous materials and obviously much cheaper.
JUST 2 DAYS AGO I CAME ACROSS A POST IN THIS COMMENTS ABOUT A RECOVERY EXPERT WHO DID A GREAT JOB FOR SOME LADIES HERE
JUST YESTERDAY I TEXTED THIS RECOVERY EXPERT FOR ASSISTANCE WITH MY STOLEN USDT. ITS LESS THAT 48 HOURS AND I ALREADY GOT RESULTS FROM HIM. IM HERE TO SAY COINSRECOVERYWORLDWIDE IS LEGITIMATE AND HELPED ME SWIFTLY WITH MY USDT RECOVERY!!!!!!! TO WHOEVER IS IN NEED OF RECOVERY REACH OUT TO HIM [COINSRECOVERYWORLDWIDE@GMAIL.COM]
ARE YOU A VICTIM OF INVESTMENT OR NFT SCAM? DO YOU WANT TO INVESTIGATE A CHEATING SPOUSE? DO YOU DESIRE CREDIT REPAIR (ALL BUREAUS)? SCHEDULE A MEETING WITH AN ETHICAL HACKER ASAP TO GET STARTED.
Let us show you the art of Ethical Hacking….!
EMERALD HACKS is a financial regulator, PRIVATE investigation and funds recovery body. We specialize in cases as regards ETHICAL HACKING, CRYPTOCURRENCY, FAKE INVESTMENT SCHEMES and RECOVERY SCAM. We are also experts in CREDIT REPAIR, we analyze what’s impacting your score.
All software tools needed to execute RECOVERIES from start to finish are available in stock.
Kindly NOTE that the available tools does NOT apply to CREDIT FIX.
Be ALERT to FALSE reviews and testimonies on the internet, the authors and perpetrators unite to form a syndicate.
Contact our team as soon as you can via the email address below to book a mail meeting with an ethical hacker.
emeraldhacks(.)org@gmail(.)com
Stay Safe out there !
WORKING WITH OPTIMISTIC HACKER GAIUS / RESTORING LOST CRYPTOCURRENCY
It is causing victims among people. I’m thrilled to tell you about my amazing experience working with OPTIMISTIC HACKER GAIUS, a recovery company. I put $155,000 into a fictitious venture. following being a victim. I didn’t know whether I would ever see my hard-earned money again, and I was devastated. I’m glad that OPTIMISTIC HACKER GAIUS, who assisted me in getting my Bitcoin back, was discovered on Google. How OPTIMISTIC HACKER GAIUS helped me get my lost cryptocurrency back is something I will never forget. I never would have thought it was possible to recover cryptocurrency. For anyone who is in a similar situation, I suggest OPTIMISTIC HACKER GAIUS. their everlasting dedication to the welfare of their clients and their unmatched proficiency in cryptocurrency recovery. You can get in contact with them using
WhatsApp.: +1..6..0..1..4..6..0..9..4..7..7
email… optimistichackergaius @ seznam.cz
They rank is one of the best recovery companies in the worldwide
ICC RECOVERY IS A RAY OF HOPE:
As the threat of fiat currency theft continues to grow, a reliable recovery service is essential. I lost $770K to a phony platform when my trading account got compromised, and I was devastated for weeks. Working with IRON CLAD CYBER RECOVERY was a transformative experience, not only did they return the stolen assets to my wallet, they also demonstrated a level of professionalism that exceeded my expectations. I came across IRON CLAD CYBER RECOVERY through Google reviews then I gave it a thought before contacting them. It was a detailed consultation to understand the theft rate. They created a recovery plan to recover my lost passwords and seed phrases, leveraging their extensive knowledge of blockchain technology and forensic investigation skills. IRON CLAD CYBER RECOVERY has an excellent track record, countless clients have shared experiences with them, highlighting the team’s professionalism, knowledge and commitment to helping customers recover from Fiat related issues. They care about the safety of their clients and uses cutting-edge technology and a knowledgeable team of experts to effectively recover stolen assets. They have built a solid reputation in the fiat recovery market due to their expertise and track record. Kindly contact IRON CLAD CYBER RECOVERY for timely recovery.
Email Address: Iron clad cyber @techie .com, WhatsApp: +1-6-2-3-6-8-8-8-8-1-5