Scientists are busy cracking the code on the next generation of battery technology. Since battery storage is incredibly important for many of the advances we are looking forward to in Industry 4.0, we will continue to develop this subject.
With the advent of more specialized mobile devices than one can even list in a short article, batteries and the technology surrounding them are currently of prime importance. We want better batteries for many reasons, including longer phone life, more efficient use of renewable energy, and even electric cars (even the flying ones).
So when a story comes across my desk about a solid-state lithium-ion battery that can, at least in theory, completely outdo the kinds of batteries we use today in almost every way, it grabs my attention.
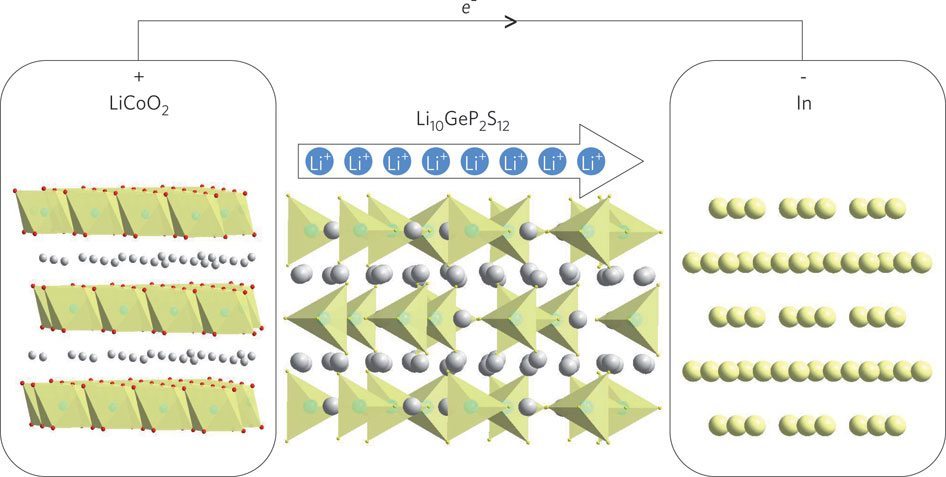
#solid #lithium #batteries are more efficient than their #liquid counterparts.Click To TweetToday’s batteries use two solid layers called electrodes that are separated by a polymer membrane infused with a liquid electrolyte.
These liquid-state lithium-ion setups have enabled so many of our high-tech devices that it is hard to imagine that they may soon be old news, and yet if you were to look at this week’s issue of Advanced Energy Materials, you would see that lithium-ion may soon switch out its liquid components for solid ones.
Making a Better Lithium Battery
So, liquid state batteries are great. Let’s start there. Without them, we may still be stuck in the dark ages when a younger me was hitting the side of my brick-sized Game Boy in the hopes that it would squeeze just a little bit more life out of the four AA’s that powered it.
Little me had no luck with that, but if you would have told me that I would be ingraining a recharging habit into his routine to power a device not even a third of the size and exponentially more powerful, he probably would have said something along the lines of “Gosh, the future sure sounds great.”
It is great. We love it here in the future. The problem is, it can get better, we know that it can get better, and we’re always on the lookout for how to make it better.
Yeah, I can use my iPhone like it’s a gaming device and never have to switch out a battery (or lose the battery cover), but I can’t do so all day. Otherwise, I’m left stranded in case of emergencies and bored out of my mind otherwise.
The power limits of liquid-state batteries notwithstanding, they also carry the small but ever-present risk of potential flammability.
When the lithium ions pass back and forth through the liquid electrolyte, there is a chance it will explode, which is managed for the most part and can be catastrophic when it does occur (we’re looking at you, Samsung Note 7). Don’t worry too much–you’re more likely to have a short-circuit than some kind of explosion, but at the end of the day, the liquid-state setup has the potential to produce tiny projections called dendrites within the electrolyte layer, which is never good for the battery in question.
By contrast, solid-state batteries could provide enormous advantages regarding energy storage, and they could eliminate the risk of forming dendrites completely. What’s more, they could lead to even smaller and lighter batteries, which would save up more room in your devices for any other fancy feature that a developer wants to add in.
It all sounds great, right? It is, but scientists are still a ways off from putting this kind of technology in your pocket. Before we start switching over to solid-state batteries, we need to learn more about their mechanical properties.
What we Need to Learn
We all remember the Note 7 from my earlier jab, or more likely because they literally blew up in people’s faces.
The reason for that explosive reaction was that the casings for their batteries did not adequately account for the natural swell and contract that occurs in the electrodes as the lithium ions pass in and out of their crystal structure. By all accounts, that swelling and contracting will still occur in solid-state batteries, and this is where scientists are focusing their research.
The more they know about a solid-state electrode’s mechanics, the more they can account for the swelling and contracting. Also, they need to be on the lookout for those aforementioned dendrites, because they could still be a damaging factor in a solid-state battery.
The trick is in finding a material that is neither too stiff or too brittle, as either one could lead to cracks that severely hamper the battery’s performance. Until now, the sensitivity of their materials to laboratory air has made it difficult to measure the mechanical properties of a solid-state battery, but the research team in this latest study got around that by submerging the battery in a bath of mineral oil. This bath not only gave the battery a decent spa-day, but it also allowed the research team to study it without any pesky chemical interactions from air or moisture.
Using this technique, the team was able to get detailed measurements for just one candidate of a lithium-conducting sulfide material that could be a likely choice for solid-state batteries.
Other researchers were keen to test the elastic properties of the material, but by using a fine-tipped probe and their mineral oil bath, the new team was able also to test its fracture properties. The resulting information gave scientists a more complete picture of how much stress the material can take, making it possible to design solid-state battery systems around that data.
Sadly, the material they used wasn’t ideal for battery use, but their methodology is sound, so they may yet find the right material to make the next generation of batteries.


















Comments (0)
Most Recent