Molecular explosions are spectacular phenomena that show the dynamics of matter under extreme conditions. To understand rock transformation under intense pressure and heat, a team of researchers at Stanford University used focused X-rays to heat up samples.
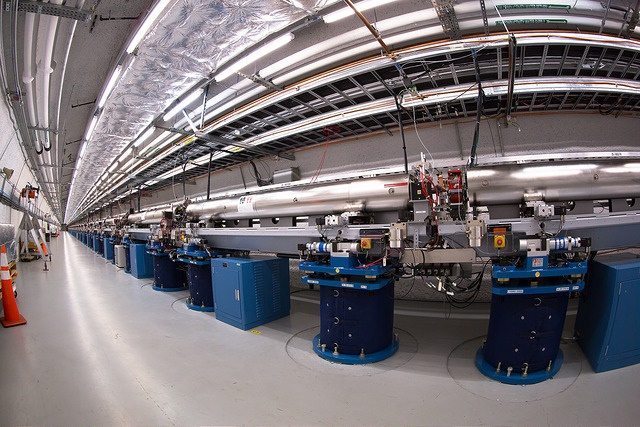
Focused X-ray pulses on a nanosecond scale have the advantage of creating controlled explosions in materials and reveal the dynamics of molecular transformation. Two researchers at Stanford University used the LCLS, a powerful X-ray laser installed in the Stanford Linear Accelerator tunnel (SLAC), to superheat a group of atoms in rock samples in less than a billionth of a second.
The Linac Coherent Light Source (LCLS)
The LCLS is to atomic physics, chemistry, and biology what CERN’s Large Hydron Collider is to particle physics. The principle of the Linac Coherent Light Source stems from the techniques used in all large particle accelerators. Other than exposing matter to superheat, physicists also use the LCLS as a kind of ultra-fast camera to monitor the evolution of quantum phenomena.
Focused X-rays to Superheat Rock Sample
Wendy Mao, an associate professor of geological sciences and photon science, and Arianna Gleason, a postdoctoral researcher at Los Alamos National Laboratory, used the LCLS X-ray laser at SLAC National Accelerator Laboratory to witness how rocks transform under extreme conditions.
“For the first time, we can begin to unravel the ultrafast transformation of a rock sample during a dynamic process like shock compression.” Said Mao, “By taking a series of snapshots, we can capture what is happening during very rapid processes.”
Subjected to laser’s extreme heat, atoms produce plasma that blows off and leaves behind a moving shockwave. This rearranges the rock sample’s atoms, turning it to a different mineral. At nanosecond intervals, scientists fire pulses of focused X-ray beams at the rock sample to witness the mineral transformation.
Exploiting the reactions to the LCLS, Mao and Gleason showed that silica transforms to the rare mineral stishovite in billionths of a second, which is much faster than previously thought.
Mao and Gleason showed that silica transforms to the rare mineral stishovite in billionths of a secondClick To Tweet“The high-pressure behavior of silica has been studied extensively because of its application not only to planetary science but fundamental physics, chemistry and materials science as well,” said Mao. “This study provides critical insight into the mechanism behind how different forms of silica transform from one structure to another.”


















Investment is one of the best ways to achieve financial freedom. For a beginner there are so many challenges you face. It’s hard to know how to get started. Trading on the Cryptocurrency market has really been a life changer for me. I almost gave up on crypto at some point not until saw a recommendation on Elon musk successfully success story and I got a proficient trader/broker Mr Bernie Doran , he gave me all the information required to succeed in trading. I made more profit than I could ever imagine. I’m not here to converse much but to share my testimony; I have made total returns of $10,500.00 from an investment of just $1000.00 within 1 week. Thanks to Mr Bernie I’m really grateful,I have been able to make a great returns trading with his signals and strategies .I urge anyone interested in INVESTMENT to take bold step in investing in the Cryptocurrency Market, he can also help you RECOVER your lost/stolen Cryptocurrencies. you can reach him on WhatsApp : + 1 (424) 285 – 0682 or his Gmail : BERNIEDORANSIGNALS @ GMAIL . COM bitcoin is taking over the world, tell him I referred you